Chemistry questions and answers. For now we will focus on the three cubic unit cells.
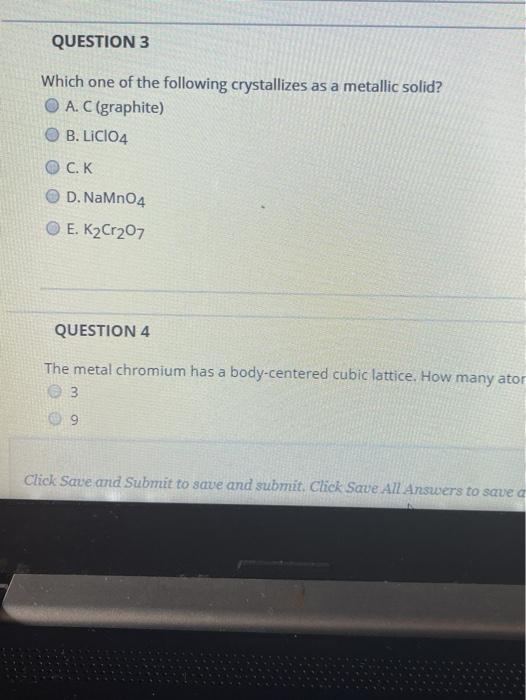
Solved Question 3 Which One Of The Following Crystallizes As Chegg Com
Exhibit 11-4Consider Aluminum metal that crystallizes in a face centered cubic cell to answer the following problem s.

. B When the solids are hit with a hammer W flattens out X shatters into many pieces Y is smashed into a powder and Z is not affected. A metallic crystal crystallizes into a lattice containing a sequence of layers AB AB AB. The edge of the unit cell is 408 pm.
A metal crystallizes in the face-centered cubic FCC lattice. Which one of the following crystallizes in a metallic lattice. NaMnO 4 K 2.
X Y and Z are poor electrical conductors. Chromium metal crystallizes as body-centered lattice. Calculate the change in enthalpy for the following process.
Question 6 0 point Which one of the following crystallizes in a metallic lattice. How many copper atoms are in each unit cell. Ratings 92 12 11 out of 12 people found this document helpful.
Solution for Metallic strontium crystallizes in a face-centered cubic lattice. 342014 21404 AM Location. If the atomic radius of Cr is 125 Å what is the density of Cr metal in gcm3.
Which one of the following crystallizes in a metallic lattice. Any packing of sphere leaves out voilds in the latticeThe percentage by. Course Title ELECTRICAL ELEC 345.
The volume of the unit cell is 225 10⁸ pm³. What is the density of strontium. Its density is 120 gcm3at 27 C.
Chemistry questions and answers. A metal crystallizes with a face centred cubic lattice. Which one of the following crystallizes in a metallic lattice.
Which crystallizes in a metallic latticeNaMnO4KCLiClO4K2Cr2O7 Get 15 discount on your first 3 orders with us Use the following coupon FIRST15 Order Now. Any packing of spheres leaves out voids in the lattice. Which one of the following crystallizes in a metalliclatticeCNaMnO4KLiClO4K2Cr2O7I think metallic.
The closest distance between two gold atoms is. Which one of the following crystallizes in a metallic lattice. Question 6 0 point which one of the following.
Calculate the number of atoms per unit cell. Which one of the following crystallizes in a metallic. Question 7 4 point Copper has a face-centered cubic unit cell.
Note that there are actually seven different lattice systems some of which have more than one type of lattice for. It crystallizes with a cubic unit cell with an edge length of 2867 pm. She is told that the solids could be gold lead sulfide PbS quartz which is SiO2 and iodine.
Nickel rhodium copper silver. All have a metallic luster. Calculate the atomicradius of Pd.
Which of the following is most likely an ionic solid. Which one of the following crystallizes in a metallic lattice. The results of her investigations are.
Click here to get an answer to your question Which one of the following crystallizes in a metallic latticeaCbNaMn04cKdLiClO4eK2Cr207. Calculate the mass of one metal atom. A metallic element crystallizes into a lattice contained sequence of layers.
The length of the cubic unit cell is 4242 A. The diameter of the metal atom is. Which of the following crystallizes in a metallic lattice.
P4O10 s -3110 kJmol H2O l -286 kJmol H3PO4 s -1279 kJmol. Simple cubic which we have already seen body-centered cubic unit cell and face-centered cubic unit cellall of which are illustrated in Figure 5. Which one of the following crystallizes in a metallic lattice.
A certain solid metallic element has a density 787 gcm3 and a molar mass of 5585 gmol. Na2Cr2O7 KMnO4 Si LICIO3 Rb. GENERAL CHEMISTRY 1 TOPIC 11 TEST Which one of the following crystallizes in a metallic lattice.
Xenon crystallizes in FCC lattice and the edge of the unit cell is 6 2 0 p m then the radius of xenon atom is. Most metal crystals are one of the four major types of unit cells. Consider the following standard heats of formation.
The density of the metal is 10500 kgm and the length of a unit cell edge a is 4086 pm. Palladium crystallizes in a face-centered cubic unit cell. A W is a good electrical conductor.
NaMnO 4 K 2 Cr 2 O 7 LiClO 4 K correct answer C. Calculate the amount of heat that must be absorbed by 100 g of ice at -20 C to convert it to liquid water at 600 C. C 10 H 8.
8 2 6 4. Which one of the following crystallizes in a metallic lattice. Chemistry questions and answers.
This preview shows page 7 - 10 out of 26 pages. Click hereto get an answer to your question Metallic gold crystallizes in fcc lattice.
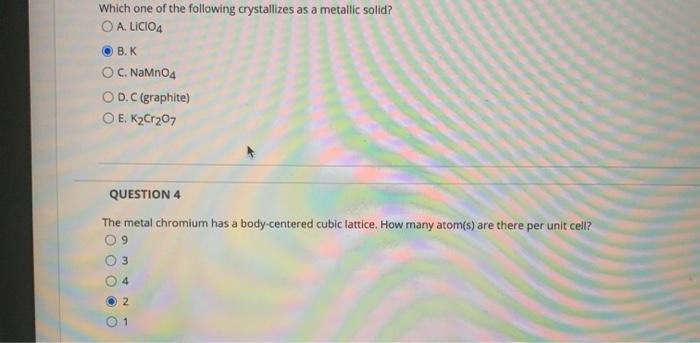
Solved Which One Of The Following Crystallizes As A Metallic Chegg Com

Solved Which One Of The Following Crystallizes In A Metallic Chegg Com

Solved 6 Which One Of The Following Crystallizes In A Chegg Com
0 Comments